Dr Rick Thompson, Dr Michael Wright and Prof Mike Briggs introduce MCDS-Therapy.
Who we are
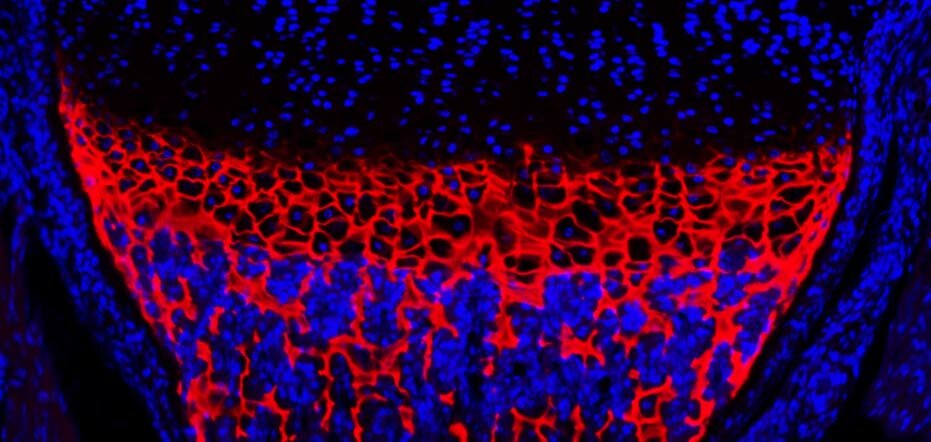
MCDS-Therapy is an EU-funded project. The MCDS-Therapy consortium is comprised of 11 international partners, who are working to develop the first therapy for the ultra-rare bone disease, metaphyseal chondrodysplasia type Schmid (MCDS).
We are running a clinical trial as part of the project to test whether the drug carbamazepine can be repurposed to treat MCDS patients. We are proving that carbamazepine is not only safe to use, but is also effective in reducing the pain and mobility difficulties experienced by MCDS patients.
Explore our website to learn more about the science behind MCDS-Therapy and subscribe to our newsletter to stay informed about our project and clinical trial. If you have any questions, please don’t hesitate to get in touch.
Watch our welcome video!
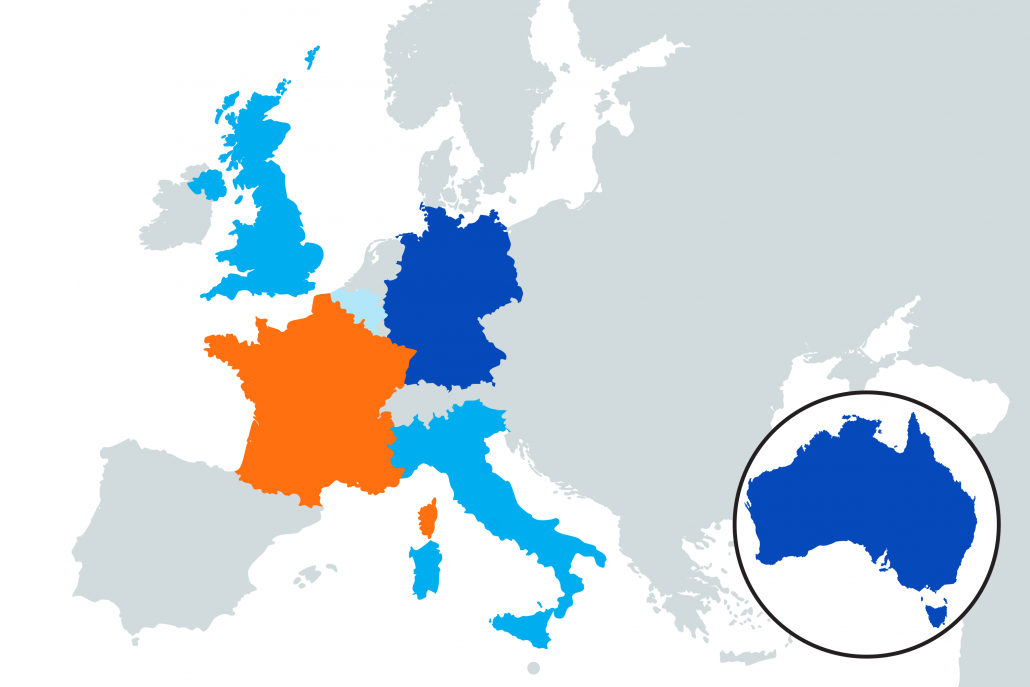
Our international sites are opening for recruitment soon!